The role of coolant in internal combustion engines
There are a lot of components in our car that we rarely pay attention to, in fact, many users never come across it, even though it has its own service interval and even its functions are not negligible.
This is the same situation in case of the coolant of water-cooled engines. In this article, we show you how traditional distilled water differs from coolant.
What is coolant?
Refrigerant is made up of two things: water and antifreeze.
Antifreeze is an additive that lowers the freezing point of a water-based liquid. An antifreeze compound is used to lower the freezing point in cold environments. Standard antifreezes also increase the boiling point of the fluid which allows for higher coolant temperatures. Antifreeze additives have a lower heat capacity than water and reduce the effectiveness of water as a coolant.
Water has good properties as a coolant, this is the reason why water and antifreeze are also used in internal combustion engines and other heat transfer applications such as HVAC coolers and solar water heaters. The purpose of the antifreeze is to prevent the hard casing from cracking as a result of the expansion which occurs when the water freezes. In the trade, both the additive (pure concentrate) and the mixture (diluted solution) are called antifreeze, depending on the context.
Careful choice of antifreeze allows for a wide temperature range in which the mixture remains in the liquid phase which is critical for efficient heat transfer and proper operation of heat exchangers.
Application in the automotive industry
Most engines are “water” cooled to remove waste heat, although “water” is actually a mixture of water and antifreeze as explained earlier. The term engine coolant is widely used in the automotive industry to cover the primary function of convective heat transfer in internal combustion engines.
In automotive applications, corrosion inhibitors are added to protect vehicle radiators, which often contain electrochemically incompatible metals (aluminum, cast iron, copper, brass, solder, etc.).
Antifreeze was developed to eliminate the deficiencies of water as a heat transfer fluid.

Coolant in expansion tank (source: www.wikipedia.org)
On the other hand, if the engine coolant gets too hot, it can boil inside the engine, creating voids (vapor pockets) that can lead to localized hot spots and fatal engine failure.
If plain water were used as engine coolant, it could freeze in many climates, causing significant internal engine damage. In addition, clean water would increase the occurrence of galvanic corrosion. The right engine coolant and pressurized coolant system will eliminate these deficiencies.
With the right antifreeze, the engine coolant can tolerate a wide temperature range.
Early antifreeze for engine coolant was methanol (methyl alcohol). Ethylene glycol was developed because its higher boiling point was more compatible with heating systems.
Coolant types
Ethylene glycol
Most antifreezes are made by mixing distilled water with additives and a base product, usually MEG (monoethylene glycol) or MPG (monopropylene glycol). Ethylene glycol solutions first became available in 1926 and were marketed as “permanent antifreeze” because the higher boiling points provided advantages in both summer and cold weather. Today, they are used in many applications, including automobiles but there are alternatives made with propylene glycol with lower toxicity.
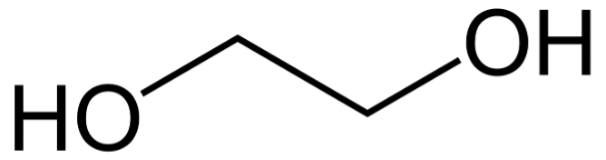
Ethylene glycol (source: www.wikipedia.org)
When ethylene glycol is used in a system, it can be oxidized to five organic acids (formic acid, oxalic acid, glycolic acid, glyoxalic acid, and acetic acid). Inhibited ethylene glycol antifreeze blends are available with additives that buffer the pH and alkalinity of the solution to prevent oxidation of the ethylene glycol and the formation of these acids. Nitrites, silicates, borates and azoles can also be used to prevent the corrosive effect of metals.
Ethylene glycol has a bitter aftertaste, but is also sweet and causes intoxication. The toxic effects of ingesting ethylene glycol occur because the liver converts it into 4 other chemicals that are much more toxic. A lethal dose of pure ethylene glycol of 1.4 mL/kg (90 mL) is lethal to a 140 lb (64 kg) person, but is much less lethal if it is treated within an hour.
Propylene glycol
Propylene glycol is significantly less toxic than ethylene glycol, essentially a “non-toxic antifreeze”. It is used as an antifreeze where ethylene glycol is not suitable, such as in food processing systems or home plumbing where for example it may be accidentally ingested by a child.
Propylene glycol is permitted in many countries to be added to many processed foods, including ice cream, frozen pudding, salad dressings, and baked goods and is commonly used as the main ingredient in “e-liquid” in electronic cigarettes. Propylene glycol is oxidized to lactic acid.
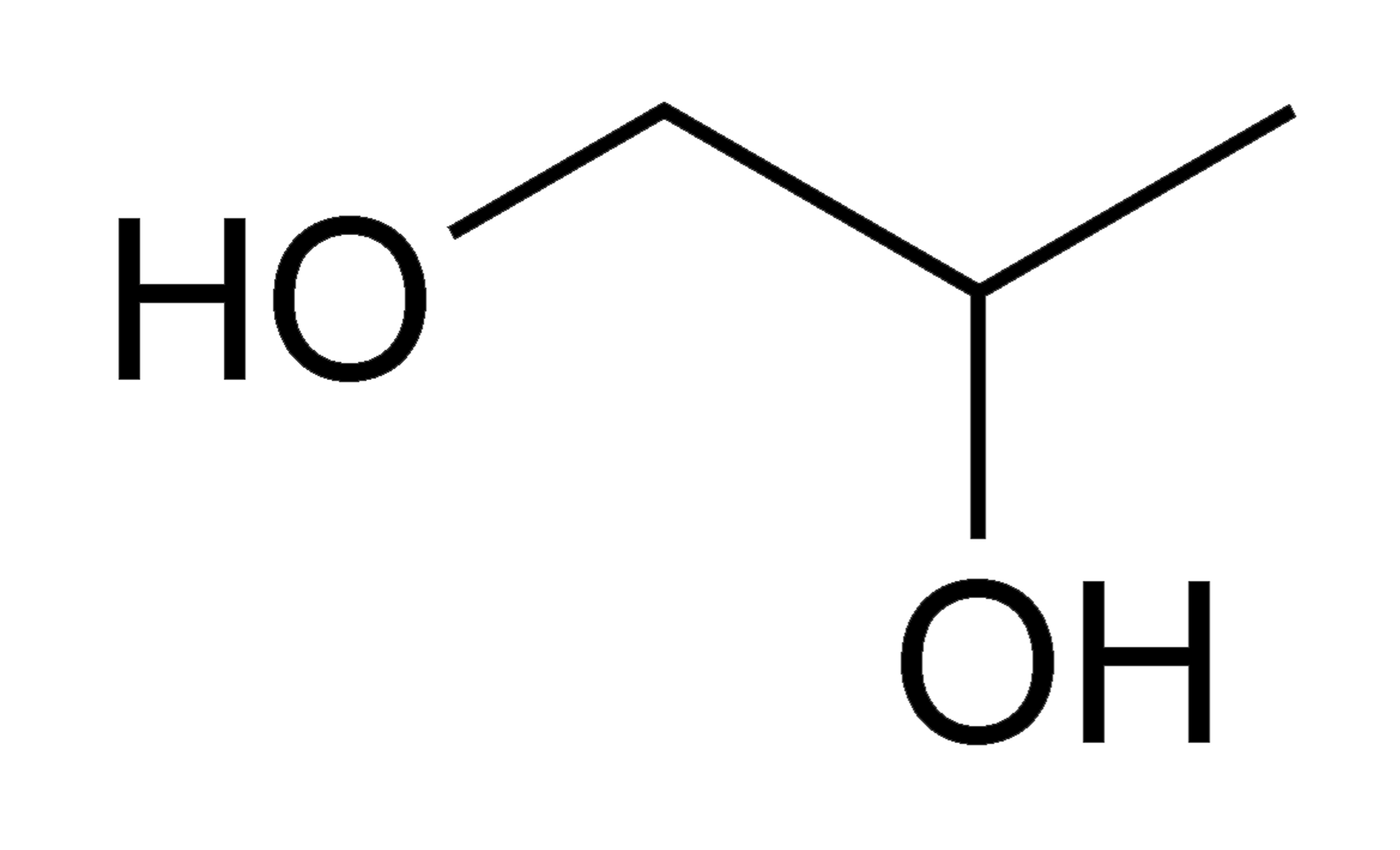
Propylene glycol (source: www.wikipedia.org)
In addition to corrosion of the cooling system, biological contamination also occurs. As the bacterial sludge begins to grow, the corrosion rate of the system increases. Maintenance of systems using glycol solution includes regular monitoring of antifreeze, pH, specific gravity, inhibitor level, color and biological contamination.
Propylene glycol should be replaced when it becomes reddish in color. If an aqueous solution of propylene glycol in a cooling or heating system turns reddish or black, this indicates that the iron in the system is corroding significantly. In the absence of inhibitors, propylene glycol can react with oxygen and metal ions to form various compounds, including organic acids (for example, formic acid, oxalic acid, acetic acid). These acids accelerate the corrosion of metals in the system.
Other common coolants
Propylene glycol methyl ether is used as an antifreeze in diesel engines. More volatile than glycols.
The advantage of glycerin used in automotive antifreezes is that it is non-toxic, resistant to relatively high temperatures and non-corrosive. But it is not widely used.
Glycerin was previously used as an antifreeze in automotive applications before being replaced by ethylene glycol. In 2008, Volkswagen introduced G13 (TL 774-G) antifreeze containing glycerin which was marketed as a more environmentally friendly product due to its low toxicity and reduced CO2 emissions. Since 2018 they have switched to G12EVO (TL 774-L), which no longer contains glycerin.